The Best CRM for Medical Device Sales
VALiNTRY360's HIPAA-certified consultants help you empower Medical Device Sales Teams with a CRM that Works for MedTech
Can Your MedTech CRM Compete?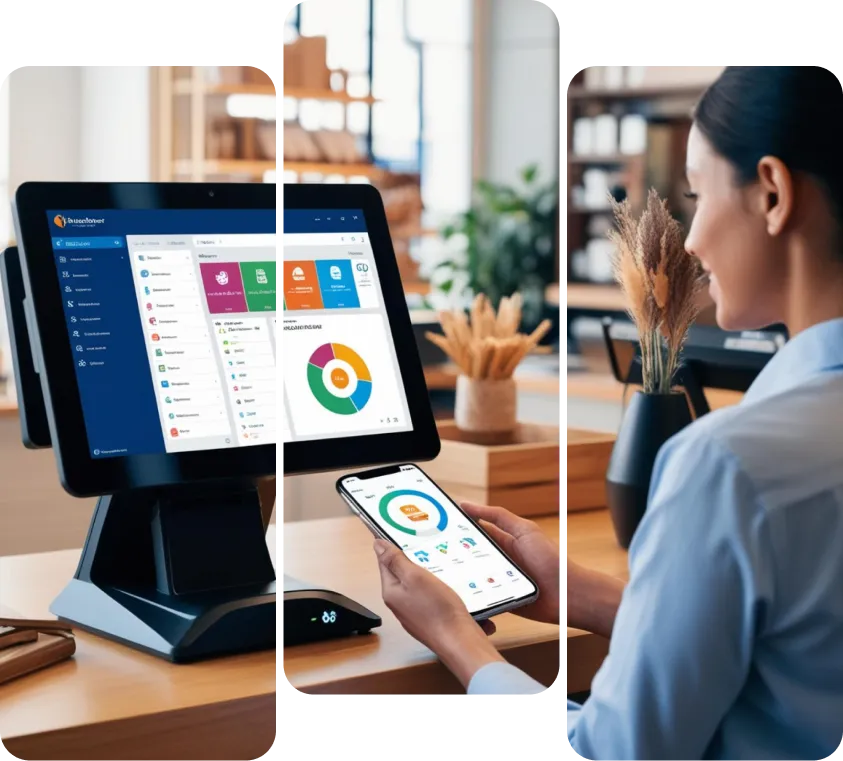

Why Your Teams Need the Best CRM for Medical Device Sales
Sales managers in medical device companies face mounting pressure to exceed revenue targets while lacking the CRM visibility needed to effectively manage complex healthcare sales cycles. Most medical device sales teams struggle with CRMs that weren't designed for healthcare complexity, forcing representatives to work around system limitations instead of focusing on relationship building with healthcare professionals. Critical opportunities slip through the cracks when CRM systems fail to capture multi-stage approval processes, clinical evaluation periods, and the complex stakeholder dynamics inherent in medical device sales.
With the global medical device industry growing at 6.5% annually and expected to expand by 63% through 2032, having the right CRM strategy is critical for capturing market share. Companies that implement properly configured CRM solutions see significant improvements in sales efficiency and achieve an average ROI of $8.71 for every dollar invested. The key is working with specialists who understand both healthcare workflows and the unique demands of medical device sales.
Transform Your Medical Device Sales ProcessAre These Challenges Costing You Revenue?

Lengthy and Complex Sales Cycles
Medical device sales often involve extended buying cycles due to the need to engage multiple stakeholders clinicians, procurement teams, and financial decision-makers. Each must evaluate the product for safety, efficacy, and ROI, which can delay deals. The complexity is further compounded by the need for clinical trials or peer-reviewed data to justify purchases, making speed-to-revenue a persistent challenge.


Limited Market Access
Expanding into new regions or healthcare networks requires navigating complex payer systems, securing reimbursement, and meeting local compliance standards. Even within existing markets, gaining placement on hospital formularies or approval from value analysis committees can be slow and uncertain. Without clear economic and clinical value propositions, market penetration becomes a major hurdle.


Intensifying Competition
The medical device market is saturated with both legacy players and new innovators. As soon as a company brings a breakthrough product to market, competitors often respond quickly with similar offerings, eroding differentiation and pricing power. To maintain growth, companies must continuously innovate and defend their market position with strong branding and evidence-based performance.


Evolving Customer Expectations
Healthcare buyers increasingly expect more than just a high-quality device they demand data-driven results, digital integration, and ongoing support. Clinicians and procurement teams want tailored experiences, educational content, and performance insights. Companies relying solely on traditional sales tactics risk falling behind as expectations shift toward personalized and value-driven engagements.


Inefficient Sales and Marketing Alignment
Many medical device companies struggle with siloed teams and disconnected systems that hinder coordination between sales and marketing. Without a unified view of the customer journey, sales reps lack insight into buyer readiness, and marketing cannot measure campaign effectiveness. This misalignment leads to missed opportunities and inconsistent revenue performance.


Low Visibility into Pipeline and Performance
Lack of centralized, real-time data on leads, opportunities, and sales outcomes makes it difficult for leadership to forecast accurately or make data-driven decisions. Legacy systems or fragmented CRMs contribute to blind spots in the pipeline, making it hard to identify what’s working and where resources should be focused to accelerate growth.

Regulatory Hurdles
Medical device companies operate under strict regulatory frameworks like the FDA in the U.S. and MDR in Europe. These requirements demand extensive documentation, testing, and post-market surveillance, which delay product launches and increase development costs. Staying compliant while accelerating time-to-market is a difficult balance that directly impacts growth potential.
Why Choose  for the Best CRM for Medical Device Sales?
for the Best CRM for Medical Device Sales?

Medical Device & Life Sciences Specialization
- VALiNTRY360's HIPAA-certified MedTech consultants bring deep expertise in sales processes, regulatory compliance, and healthcare relationship management with 150+ years of HIPAA-compliant, HITRUST certified healthcare experience.
- We help med-tech firms rapidly adapt to new technologies, integrating AI and IoT for innovative development while ensuring regulatory compliance across FDA and CE regulations as your trusted medical technology consultant.

Certified Salesforce-Branded Services Partner
- As a certified Salesforce-Branded Services Partner, VALiNTRY360 offers specialized Life Sciences implementations with certifications spanning Salesforce Health Cloud, Sales Cloud, Service Cloud, and Life Sciences Cloud.
- We deliver the best CRM for medical device sales through proven Salesforce CRM for healthcare architectures that support complex product catalogues, clinical data integration, multi-channel healthcare professional engagement, and end-to-end patient care workflows.

Proven Client Success in MedTech
- Our MedTech healthcare consultants consistently deliver exceptional results, with medical device companies implementing our Health Cloud solutions experiencing improved sales productivity, enhanced regulatory compliance, and accelerated revenue growth.
- Our expertise extends beyond technical implementation to include change management, user adoption strategies, and ongoing optimization to ensure long-term success of your medical sales software investment.
Our Comprehensive Medical Device Salesforce Solutions
VALiNTRY360 delivers end-to-end Salesforce consulting services specifically designed for medical device companies, addressing every aspect of your sales, marketing, and customer relationship management needs:
- Healthcare Professional 360-Degree View: Create unified profiles combining clinical interests, prescribing patterns, research activities, and purchasing history to enable personalized sales approaches and omni-channel nurturing campaigns
- Medical Device Inventory Management: Real-time tracking of device availability, lot numbers, expiration dates, and usage patterns across multiple facilities with automated demand forecasting, inventory alerts, and proactive resupply triggers
- Clinical Trial Integration & Device Monitoring: Seamlessly connect clinical research data with commercial activities, enabling evidence-based sales conversations, while integrating device status triggers to proactively address maintenance issues before downtime impacts patient care
- Complex Product Configuration & Revenue Intelligence: Advanced product catalog management supporting device families, accessories, and service contracts with dynamic pricing models, automated quantity discounts, and machine learning-powered forecast accuracy
- Healthcare Account & Territory Management: Map complex hospital networks, physician groups, and GPOs to optimize territory planning and account penetration, with automated commission payments and intelligent territory distribution
- Streamlined Quoting & Contracting: Accelerated quote generation with regulatory-compliant documentation, automated approvals, procurement system integration, and enhanced sales processes that elevate lead generation and strategic market insights
- HIPAA-Compliant Data Handling: Salesforce Shield ensures the utmost security and compliance when handling sensitive PHI (Protected Health Information) data with comprehensive audit trail documentation
- Adverse Event Management: Automated tracking and reporting of product-related adverse events, complaint handling, and regulatory submission workflows with faster detection and reporting capabilities
- FDA and CE Regulation Compliance: Robust tools for regulatory compliance ensuring adherence to FDA and CE regulations with real-time monitoring, actions, and quality process management
- Medical Affairs & Evidence-Based Marketing: Bridge clinical and commercial teams through shared platforms that track medical education, KOL management, and connect clinical research outcomes with targeted marketing campaigns
- Multi-Channel HCP Engagement: Coordinate interactions across in-person visits, digital platforms, medical conferences, and educational programs with omni-channel nurturing campaigns and clinical collaboration enhancement
- Provider, Patient & Distributor Portals: Self-service options for providers and distributors to place orders, track status, and make payments, plus personalized patient experiences with proactive care recommendations and automated communications
- Centralized Document & Order Management: Streamline quality processes for enhanced traceability, supplier collaboration, comprehensive audit trails, while holistically managing purchase orders, invoices, contracts, and shipment tracking
- System Interoperability & IoT Integration: Facilitate seamless integration for enhanced stakeholder communication, improving patient outcomes through 3rd party integrations and rapid AI/IoT technology adoption
- Consumables Management: Automate refill schedules, shipping logistics, and delivery for consumable products to improve device uptime, minimize care delivery gaps, and ensure optimal inventory levels
Technical Expertise for Enterprise-Grade MedTech CRM Solutions
Our certified Salesforce Health Cloud architects design enterprise-grade solutions that handle complex medical device ecosystems with millions of patient interactions while maintaining 99.9% uptime and strict HIPAA compliance. Medical device companies operate an average of 1,061 different applications across their enterprise, with only 29% properly integrated, creating critical gaps in patient care coordination and sales visibility.
VALiNTRY360’s proven methodologies ensure seamless data flow across disparate healthcare platforms while maintaining enterprise security, regulatory compliance, and scalability requirements. Our technical implementations integrate clinical systems, inventory management platforms, regulatory databases, and customer engagement tools into unified Salesforce environments that support both immediate business needs and long-term growth strategies.
Our Proven Medical Device Salesforce Implementation Process
We follow a specialized methodology designed specifically for medical device companies to ensure successful Health Cloud deployments that drive immediate business value:

Step 1: Specialized Medical Device Sales Workflow Assessment
- Analysis of current sales processes, customer journeys
- Detailed mapping of Compliances, clinical workflows, FDA and CE regulations
- Assessment of existing technology stack
- Evaluation of workflow management from referral-to-treatment
Step 2: Architecture Design & Customization
- Design specialized data models
- Configuration of industry-specific workflows for
- Development of custom objects for processes
- Integration architecture planning for seamless data flow
Step 3: MedTech-Focused Development & Testing
- Build specialized Health Cloud solutions
- Implementation of regulatory compliance features
- Development of mobile solutions
- Comprehensive testing including HIPAA and more
Step 4: Deployment & Training
- Phased rollout strategy minimizing disruption
- Specialized training programs for personnel
- Documentation of medical device-specific processes
- Ongoing support and optimization
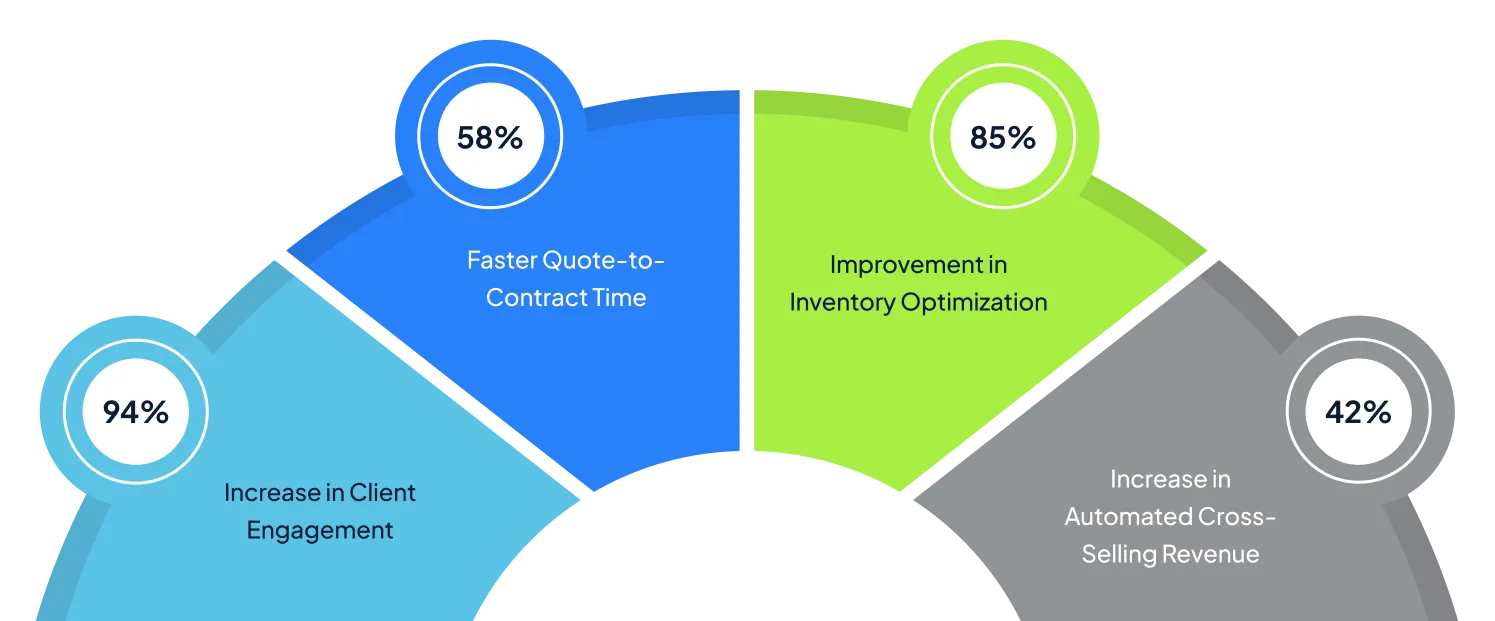
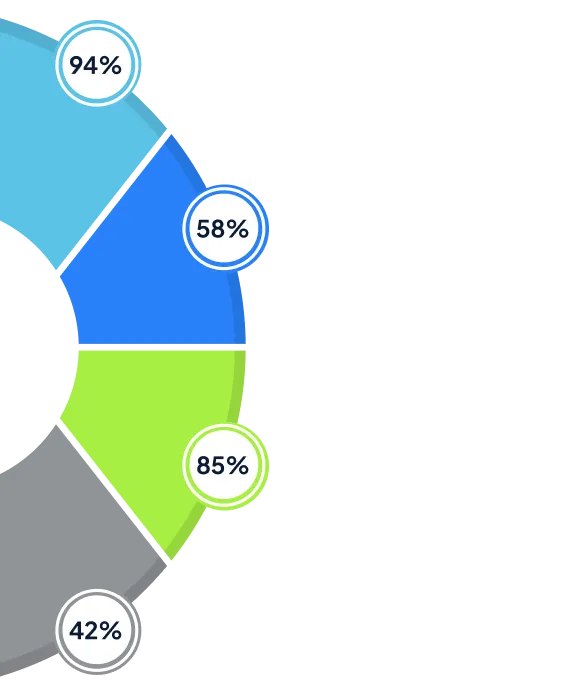
Measurable Benefits of VALiNTRY360's MuleSoft Solutions
Our clients consistently achieve significant improvements in sales performance and operational efficiency:
Medical Device Specialties We Serve
Cardiovascular Devices
Implement specialized CRM workflows for cardiac catheterization labs, supporting complex procedures, clinical outcomes tracking, and physician preference management. Our solutions integrate with cardiac imaging systems and patient monitoring platforms to provide comprehensive case management and follow-up protocols.
Orthopedic and Surgical Devices
Develop sophisticated inventory management systems that track implants, instruments, and consumables across multiple surgical suites. Our platform manages surgeon preferences, procedure scheduling, and post-operative outcomes to optimize inventory levels while ensuring immediate availability for critical procedures.
Diagnostic and Imaging Equipment
Create integrated platforms that connect equipment sales with service contracts, utilization monitoring, and clinical outcomes. Our solutions track equipment performance, manage upgrade cycles, and correlate usage patterns with customer satisfaction metrics to drive strategic account growth.

Medical Technology and Digital Health
Build unified systems connecting medical devices with software platforms, mobile applications, and cloud-based analytics. Our solutions integrate real-world evidence collection, remote monitoring capabilities, and predictive analytics to support value-based care initiatives.

Pharmaceutical and Life Sciences Integration
Develop integrated platforms supporting drug-device combinations, companion diagnostics, and personalized medicine initiatives. Our systems manage complex regulatory pathways, clinical trial coordination, and commercial launch strategies across multiple therapeutic areas.
Comprehensive Medical Device CRM Consulting Services
As the leading provider of MedTech CRM solutions for medical device companies, VALiNTRY360 delivers specialized consulting services that address every aspect of your medical device business:
Customer Data Platform for Medical Devices:
Create unified healthcare professional profiles combining clinical research, purchasing history, and engagement preferences using advanced Health Cloud capabilities
Medical Device Sales Automation
Implement automated workflows for complex medical device sales processes, from initial clinical evaluation through procurement and implementation
MedTech CRM Architecture:
Design scalable architectures supporting medical device product families, regulatory requirements, and healthcare integration standards
Healthcare Integration Expertise:
Connect Salesforce with EHR systems, clinical databases, and medical device platforms for seamless data flow and comprehensive visibility
In Their Own Words: Customer Success Story
Get Started with 
FREE Initial Consultation
Certified experts solve fragmentation and reduce technical debt
Rapid Implementation Methodology
Launch integrations quickly, boosting efficiency and responsiveness
Enterprise-Grade Solutions
Scale securely with compliant, future-ready integration architecture

Intelligent Healthcare Solutions, Exceptional Results
As a leading CRM for Medical Device Sales Service provider in the USA, VALiNTRY360 delivers comprehensive solutions that help businesses compete in rapidly changing markets. With our expert Consulting Service, we work closely with your team to implement customized strategies that drive measurable business results.
The Best CRM for Medical Device Sales FAQ by
MedTech refers to medical technology companies that develop, manufacture, and sell medical devices, equipment, and software used in healthcare settings. The global MedTech industry includes everything from simple diagnostic tools and surgical instruments to complex robotic surgery systems and AI-powered medical devices, representing a market growing at 6.5% annually.
A med tech CRM is specialized customer relationship management software designed specifically for medical technology companies’ unique needs. Unlike generic CRMs, a MedTech CRM handles complex healthcare relationships, regulatory compliance, clinical trial integration, device tracking, and multi-stakeholder hospital procurement processes that define medical device sales cycles.
Salesforce Health Cloud provides industry-specific functionality designed for healthcare relationships, regulatory compliance, and complex medical device sales processes. As the #1 CRM provider for 11 consecutive years, Salesforce offers:
- Healthcare-specific data models and workflows
- HIPAA compliance and Salesforce Shield security
- Integration with clinical systems and EHR platforms
- AI-powered insights for personalized healthcare professional engagement
- Proven ROI of $8.71 for every dollar invested
VALiNTRY360 stands out as a Salesforce-Branded Services Partner with specialized MedTech expertise:
- HIPAA-compliant, HITRUST certified healthcare3 consultants who understand medical device sales processes
- Medical technology consultant expertise spanning FDA/CE regulations
- 92% first-time implementation success rate for medical device companies
- Proven results with measurable improvements in sales productivity and compliance
Absolutely. Salesforce’s functionality addresses unique medical device sales complexity through:
- Multi-committee approval tracking across hospital networks
- Clinical evaluation period management and outcomes integration
- Healthcare account hierarchy mapping for complex organizations
- Device uptime monitoring and maintenance triggers
- Integration with clinical trials and research data
Our MedTech healthcare consultants serve companies across all segments, from startups to Fortune 500 enterprises, including:
- Cardiovascular and surgical device manufacturers
- Diagnostic and imaging equipment companies
- Orthopedic and implant device makers
- Digital health and IoMT solution providers
- Life sciences companies with device components
VALiNTRY360 delivers proven medical device-specific frameworks within 90 days through our four-phase methodology:
Phase 1: Specialized assessment and healthcare workflow analysis
Phase 2: Salesforce architecture design and customization
Phase 3: Healthcare-focused development and testing
Phase 4: Deployment and medical device team training
Our HIPAA-certified Salesforce MedTech consultants implement comprehensive security measures including:
- End-to-end encryption and field-level security
- Detailed audit logging and access controls
- Salesforce Shield for advanced PHI protection
- Role-based access controls aligned with healthcare privacy protocols
- Complete documentation for regulatory inspections
VALiNTRY360 enables seamless integration with:
- EHR systems and clinical databases
- Inventory management and supply chain platforms
- Regulatory submission and compliance systems
- Clinical trial management software
- Financial and procurement systems
- IoT devices and remote monitoring platforms
Salesforce Health Cloud provides comprehensive regulatory compliance through:
- Built-in audit trails for FDA and CE regulations
- Automated adverse event tracking and reporting
- Quality management and documentation workflows
- Real-time compliance monitoring and alerts
- Integration with regulatory submission systems
Our specialized approach connects clinical research directly with commercial activities:
- Real-time integration of trial outcomes with sales data
- Evidence-based sales conversation enablement
- Post-market surveillance automation
- Clinical performance tracking for device optimization
- Regulatory submission workflow automation
Modern medical device sales require mobile-optimized solutions. VALiNTRY360 implements:
- HIPAA-compliant mobile access for hospital environments
- Offline capabilities for areas with restricted connectivity
- Real-time CRM updates from surgical suites and clinics
- Mobile access to clinical data and device specifications
- Automated field activity capture and reporting
We offer comprehensive managed services including:
- 24/7 platform monitoring and proactive issue resolution
- Regular system optimization and performance tuning
- User adoption support and advanced training
- Compliance updates and regulatory alignment
- Strategic consulting for evolving business needs
Our solutions provide:
- Real-time device tracking across multiple facilities
- Automated inventory alerts and reorder triggers
- Lot number and expiration date management
- Usage pattern analysis and demand forecasting
Integration with supply chain and logistics systems
VALiNTRY360 provides specialized training programs covering:
- Healthcare-specific Salesforce features and workflows
- Medical device sales process optimization
- Compliance and regulatory requirements
- Mobile CRM usage in healthcare environments
- Change management for user adoption success
Modern medical device companies must demonstrate clinical outcomes and economic value. Salesforce Health Cloud enables:
- Real-world evidence collection and analysis
- Patient outcome tracking and correlation
- Cost-effectiveness data management
- ROI demonstration for healthcare buyers
- Integration with value-based contract management
We configure solutions for various medical device sales approaches:
- Direct sales to hospitals and health systems
- Distribution channel management
- Group purchasing organization (GPO) relationships
- Value-based contracting and outcome agreements
- Consignment and vendor-managed inventory models
VALiNTRY360 combines:
- Salesforce-Branded Services Partner certification and expertise
- HIPAA-certified medtech consultants who understand your challenges
- Proven track record with 92% first-time implementation success
- Comprehensive solutions from strategy through ongoing optimization
- Industry-specific experience across medical device segments
Working with VALiNTRY360 means partnering with the medical device CRM experts who transform technology challenges into competitive advantages, ensuring your team has the tools, visibility, and compliance capabilities needed to succeed in the demanding MedTech marketplace.

 for the
for the 
